资讯中心
众巍为您提供行业第一资讯!
Wechem Newsletter 1: An Introduction Of WECHEM Boron Trifluoride
发布日期:2020-12-11
来源:
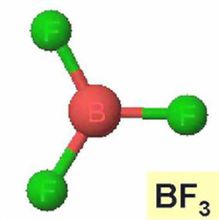
BORON TRIFLUORIDE (formula: BF3) is a non-flammable compressed gas that is used as an acidic catalyst or co-catalyst in a varying array of organic synthesis reactions. It is one of the strongest Lewis acids (an electron-pair acceptor) reacting readily with chemicals containing oxygen, nitrogen, sulfur and other electron pair donors.
Boron Trifluoride is used as a catalyst for organic synthesis reactions, for the manufacture of synthetic oils and lubricant additives and in the production of high-purity boron isotopes.
Boron Trifluoride (BF3) can be found in a number of applications. As a strong Lewis acid, BF3’s catalytic properties are used for such reactions as alkylation of aromatic hydrocarbons, polymerization of phenolic and epoxy resins as well as other isomerization, esterification, and condensation reactions. It is used as a source for the manufacture of high-purity boron isotopes such as those found in nuclear waste containment and neutron radiation control applications as well as applications in the semiconductor industry such as the manufacture of semiconductor grade silicone. BF3 is also used as a gas flux for soldering and brazing and in the production of diborane and other boron-containing compounds.

Note: Boron trifluoride is soluble in cold water, 332 g/100 ml at 0°C.
The occupational exposure limit value should not be exceeded during any part of the working exposure. Depending on the degree of exposure, periodic medical examination is suggested. The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort. Rest and medical observation are therefore essential. Immediate administration of an appropriate inhalation therapy by a doctor or a person authorized by him/her, should be considered.
Boron trifluoride (and organic complexes such as BF3-etherate) are extremely corrosive substances that are destructive to all tissues of the body. Upon contact with moisture in the skin and other tissues, these compounds react to form hydrofluoric acid and fluoroboric acid, which cause severe burns. Boron trifluoride gas is extremely irritating to the skin, eyes, and mucous membranes. Inhalation of boron trifluoride can cause severe irritation and burning of the respiratory tract, difficulty breathing, and possibly respiratory failure and death. Exposure of the eyes to BF3can cause severe burns and blindness. This compound is not considered to have adequate warning properties.
Boron trifluoride has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. Chronic exposure to boron trifluoride gas can cause respiratory irritation and damage.
Wechem is one of the leading suppliers of compressed BF3 gas to worldwide customers and markets. We are experienced to offer quick supply and superior logistics to overseas customers. Our trailers are designed and allow us to supply bulk BF3 shipments to all regions of the world.

A Brief Introduction to Wechem’s Boron Trifluoride:
1. Identification
CAS |
7637-07-2 |
Chemical formula |
BF3 |
UN Risk Classification: |
2.3 |
UN Number |
UN1008 |
United Nations Dangerous Goods Number |
328863 |
2. Specifications
Purity , % |
99.9 |
Inert gases ( N2+ O2+ CO2) |
≤400 ppmv |
SO2 ( expressed as S ) |
≤500 ppmv |
SIF4 |
≤ 10 ppmv |
3. Technical Information
Cylinder State @ 21.1°C |
Gas |
Flammable Limits In Air |
Non-flammable |
Molecular Weight (g/mol) |
67.805 |
Specific gravity (air =1) |
2.32 |
Critical Temperature ( °C ) |
-12.25 |
Critical Pressure ( psig ) |
708.32 |
CGA/DISS/JIS/DIN477 |
330/642/W22-14L/NO.8 |
Storage methods |
DOT 47L / Tube trailer |